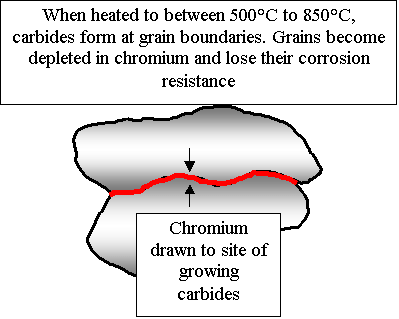
Austenitic stainless steels have high ductility, low yield stress and relatively high ultimate tensile strength, when compare to a typical carbon steel. A carbon steel on cooling transforms from Austenite to a mixture of ferrite and cementite. With austenitic stainless steel, the high chrome and nickel content suppress this transformation keeping the material fully austenite on cooling (The Nickel maintains the austenite phase on cooling and the Chrome slows the transformation down so that a fully austenitic structure can be achieved with only 8% Nickel). Heat treatment and the thermal cycle caused by welding, have little influence on mechanical properties. However strength and hardness can be increased by cold working, which will also reduce ductility. A full solution anneal (heating to around 1045°C followed by quenching or rapid cooling) will restore the material to its original condition, removing alloy segregation, sensitisation, sigma phase and restoring ductility after cold working. Unfortunately the rapid cooling will re-introduce residual stresses, which could be as high as the yield point. Distortion can also occur if the object is not properly supported during the annealing process. Austenitic steels are not susceptible to hydrogen cracking, therefore pre-heating is seldom required, except to reduce the risk of shrinkage stresses in thick sections. Post weld heat treatment is seldom required as this material as a high resistance to brittle fracture; occasionally stress relief is carried out to reduce the risk of stress corrosion cracking, however this is likely to cause sensitisation unless a stabilised grade is used (limited stress relief can be achieved with a low temperature of around 450°C ). Austenitic steels have a F.C.C atomic structure which provides more planes for the flow of dislocations, combined with the low level of interstitial elements (elements that lock the dislocation chain), gives this material its good ductility. This also explains why this material has no clearly defined yield point, which is why its yield stress is always expressed as a proof stress. Austenitic steels have excellent toughness down to true absolute (-273°C), with no steep ductile to brittle transition. This material has good corrosion resistance, but quite severe corrosion can occur in certain environments. The right choice of welding consumable and welding technique can be crucial as the weld metal can corrode more than the parent material. Probably the biggest cause of failure in pressure plant made of stainless steel is stress corrosion cracking (S.C.C). This type of corrosion forms deep cracks in the material and is caused by the presence of chlorides in the process fluid or heating water/steam (Good water treatment is essential ), at a temperature above 50°C, when the material is subjected to a tensile stress (this stress includes residual stress, which could be up to yield point in magnitude). Significant increases in Nickel and also Molybdenum will reduce the risk. Stainless steel has a very thin and stable oxide film rich in chrome. This film reforms rapidly by reaction with the atmosphere if damaged. If stainless steel is not adequately protected from the atmosphere during welding or is subject to very heavy grinding operations, a very thick oxide layer will form. This thick oxide layer, distinguished by its blue tint, will have a chrome depleted layer under it, which will impair corrosion resistance. Both the oxide film and depleted layer must be removed, either mechanically (grinding with a fine grit is recommended, wire brushing and shot blasting will have less effect), or chemically (acid pickle with a mixture of nitric and hydrofluoric acid). Once cleaned, the surface can be chemically passivated to enhance corrosion resistance, (passivation reduces the anodic reaction involved in the corrosion process). Carbon steel tools, also supports or even sparks from grinding carbon steel, can embed fragments into the surface of the stainless steel. These fragments can then rust if moistened. Therefore it is recommended that stainless steel fabrication be carried out in a separate designated area and special stainless steel tools used where possible. If any part of stainless-steel is heated in the range 500 degrees
to 800 degrees for any reasonable time there is a risk that the chrome
will form chrome carbides (a compound formed with carbon) with any carbon
present in the steel. This reduces the chrome available to provide
the passive film and leads to preferential corrosion, which can be severe.
This is often referred to as sensitisation. Therefore it is advisable
when welding stainless steel to use low heat input and restrict the maximum
interpass temperature to around 175°, although sensitisation of modern
low carbon grades is unlikely unless heated for prolonged periods.
Small quantities of either titanium (321) or niobium (347) added to stabilise
the material will inhibit the formation of chrome carbides.
To resist oxidation and creep high carbon grades such as 304H or 316H are often used. Their improved creep resistance relates to the presence of carbides and the slightly coarser grain size associated with higher annealing temperatures. Because the higher carbon content inevitably leads to sensitisation, there may be a risk of corrosion during plant shut downs, for this reason stabilised grades may be preferred such as 347H. The solidification strength of austenitic stainless steel can be seriously impaired by small additions of impurities such as sulphur and phosphorous, this coupled with the materials high coefficient of expansion can cause serious solidification cracking problems. Most 304 type alloys are designed to solidify initially as delta ferrite, which has a high solubility for sulphur, transforming to austenite upon further cooling. This creates an austenitic material containing tiny patches of residual delta ferrite, therefore not a true austenitic in the strict sense of the word. Filler metal often contains further additions of delta ferrite to ensure crack free welds. The delta ferrite can transform to a very brittle phase called sigma, if heated above 550°C for very prolonged periods (Could take several thousand hours, depending on chrome level. A duplex stainless steel can form sigma phase after only a few minutes at this temperature) The very high coefficient of expansion associated with this material means that welding distortion can be quite savage. I have seen thick ring flanges on pressure vessel twist after welding to such an extent that a fluid seal is impossible. Thermal stress is another major problem associated with stainless steel; premature failure can occur on pressure plant heated by a jacket or coils attached to a cold veesel. This material has poor thermal conductivity, therefore lower welding current is required (typically 25% less than carbon steel) and narrower joint preparations can be tolerated. All common welding processes can be used successfully, however high deposition rates associated with SAW could cause solidification cracking and possibly sensitisation, unless adequate precautions are taken. To ensure good corrosion resistance of the weld root it must be protected
from the atmosphere by an inert gas shield during welding and subsequent
cooling. The gas shield should be contained around the root of the
weld by a suitable dam, which must permit a continuous gas flow through
the area. Welding should not commence until sufficient time has elapsed
to allow the volume of purging gas flowing through the dam to equal at
least the 6 times the volume contained in the dam (EN1011 Part 3 Recommends 10). Once purging is
complete the purge flow rate should be reduced so that it only exerts a
small positive pressure, sufficient to exclude air. If good corrosion
resistance of the root is required the oxygen level in the dam should not
exceed 0.1%(1000 ppm); for extreme corrosion resistance this should be reduced to
0.015% (150 ppm). Backing gasses are typically argon or helium; Nitrogen Is often
used as an economic alternative where corrosion resistance is not critical, Nitrogrn + 10% Helium is better. A wide variety of proprietary pastes
and backing materials are available than can be use to protect the root
instead of a gas shield. In some applications where corrosion and
oxide coking of the weld root is not important, such as large stainless
steel ducting, no gas backing is used.
Carbon content: 304 L grade Low Carbon, typically 0.03% Max 304 grade Medium Carbon, typically 0.08% Max 304H grade High Carbon, typically Up to 0.1% The higher the carbon content the greater the yield strength. (Hence the stength advantage in using stabilised grades) Typical Alloy Content
All the above stainless steel grades are basic variations of a 304. All are readily weldable and all have matching consumables, except for a 304 which is welded with a 308 or 316, 321 is welded with a 347 (Titanium is not easily transferred across the arc) and a 316Ti is normally welded with a 318. Molybdenum has the same effect on the microstructure as chrome, except
that it gives better resistance to pitting corrosion. Therefore a
316 needs less chrome than a 304.
|